How a pH meter works
To be able to measure pH one needs to have a ph meter which is sensitive to the hydrogen ions that define the pH value. The principle of the measurement is that one takes a sensor with a glass membrane which is sensitive to hydrogen ions and observes the reaction between it and a sample solution. However, the observed potential of the pH-sensitive electrode alone does not provide enough information and so we need a second sensor. This is the sensor that supplies the reference signal or potential for the pH sensor.It is necessary to use the difference potential of both these electrodes together in order to determine the pH value of the measured solution. The response of the pH-sensitive electrode is dependent on the H+ ion concentration and therefore gives a signal that is determined by how acidic/alkaline the solution is.
The reference electrode on the other hand is not responsive to the H+ ion concentration in the sample solution and will therefore always produce the same, constant potential against which the pH sensor potential is measured.
The potential between the two electrodes is therefore a measure of the number of hydrogen ions in the solution, which by definition gives one the pH value of the solution. This potential is a linear function of the hydrogen concentration in the solution, which allows quantitative measurements to be made.
pH meters measure the difference between the two electrode potentials and coverts this into a pH value
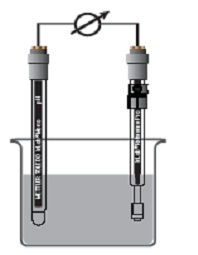 | Figure 1 shows a pH meter measurement setup with two separate sensors, a pH sensor and a reference sensor is shown. Nowadays, ph meters merge the two separate sensors into one electrode. This combination of reference and pH electrodes is called the combined pH meter electrode. | |
| Figure 1. The measurement assembly of pH and reference sensor. |
| Find out more about our range of ph meters | More advice on ph meters | |
  |  |
Source of article: A Guide to pH measurement by Mettler Toledo

