Types of pH Meter Electrode
pH Meter Electrode
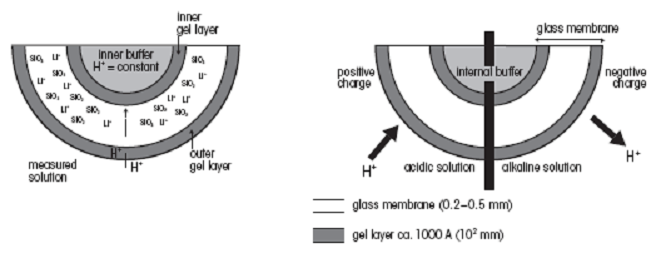
The pH
meter electrode is the part that actually senses the pH in the
solution. It consists of a glass shaft with a thin glass membrane at
the end, sensitive to H+ ions. The outside of this membrane glass forms
a gel layer when the membrane comes into contact with an aqueous
solution. A similar gel layer is also formed on the inside of the
membrane glass, since the electrode is filled with an inner aqueous
electrolyte solution. An example of this gel layer is shown in the
figure below:
 |
The
H+ ions in and around the gel layer can either diffuse into or out of
this layer, depending on the pH value and thus H+ ion concentration
of the measured solution. If the solution is alkaline the H+ ions
diffuse out of the layer and a negative charge is established on the
outer side of the membrane. Since the glass electrode has an internal
buffer with a constant pH value, the potential on the inner surface of
the membrane remains constant during the measurement. The pH meter
electrode potential is therefore the difference between the inner and
outer charge of the membrane. A drawing of a standard pH meter
electrode is shown below.
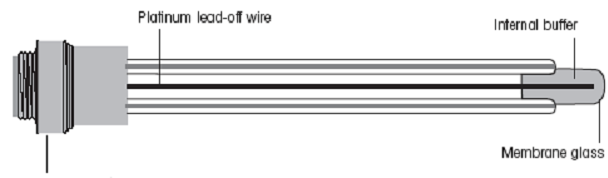
|
| pH meter electrode with pH-sensitive membrane |
Reference pH Meter Electrode
The
purpose of the reference electrode is to provide a defined stable
reference potential for the pH sensor potential to be measured against.
To be able to do this the reference electrode needs to be made of a
glass which is not sensitive to the H+ ions in the solution. It must
also be open to the sample environment into which it is dipped. To
achieve this, an opening or junction is made in the shaft of the
reference electrode through which the inner solution or reference
electrolyte can flow out of into the sample. The reference electrode
and pH half-cell have to be in the same solution for correct
measurements. A picture of a typical reference electrode is
shown below:

|
ph meter reference electrode with reference electrolyte, reference element and junction.
|
The
construction of the electrode is such that the internal reference
element is immersed in a defined reference buffer and is indirectly in
contact with the sample solution via the junction. This contact chain
ensures a stable potential. There are several reference systems
available, but the one used almost exclusively today is the
silver/silver chloride system. The potential of this reference system
is defined by the reference electrolyte and the silver/silver chloride
reference element. It is important that the reference electrolyte has a
high ion concentration which results in a low electrical resistance.
Since the reference electrolyte flows into the sample solution during
measurement, one should be aware of any possible reactions between the
reference electrolyte and the sample solution, as this can affect the
electrode and measurement.
Combined pH Meter electrodes
Combined
pH meter electrodes are much easier to handle than two separate
electrodes and are very commonly used today. In the combined electrode
the pH-sensitive glass electrode is concentrically surrounded by the
reference electrode filled with reference electrolyte. The separate pH
and reference parts of the combined electrode have the same properties
as the separate electrodes; the only difference is that they are
combined into one electrode for ease of use. Only when the two
components of the combined electrode are expected to have very
different life expectancies is the use of individual pH and reference
electrodes recommended rather than a single combined electrode. To
further simplify pH measurements, one can house a temperature sensor in
the same body as the pH and reference elements. This allows temperature
compensated measurements to be made. Such electrodes are also called
3-in-1 electrodes.

|
Typical combination pH meter electrode with inner pH sensor and outer reference element.
|
| Find out more about our range of ph meters | | More advice on ph meters |

 | |  |
Source of article: A Guide to pH measurement by Mettler Toledo








